Does Sublimation Release Thermal Energy . Yes, sublimation involves the absorption of energy. The solid phase is at a lower energy. usually the change occurs when adding or removing heat at a particular temperature, known as the melting point or. the direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. sublimation is the transfer of molecules from the solid phase to the gas phase. does sublimation involve the release of energy? sublimation, in physics, conversion of a substance from the solid to the gaseous state without its becoming liquid. An example is the vaporization of frozen. however, at −78.5 °c, the surface molecules acquire enough thermal energy to completely overcome the attractive forces and.
from www.chegg.com
Yes, sublimation involves the absorption of energy. An example is the vaporization of frozen. usually the change occurs when adding or removing heat at a particular temperature, known as the melting point or. the direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. does sublimation involve the release of energy? however, at −78.5 °c, the surface molecules acquire enough thermal energy to completely overcome the attractive forces and. The solid phase is at a lower energy. sublimation is the transfer of molecules from the solid phase to the gas phase. sublimation, in physics, conversion of a substance from the solid to the gaseous state without its becoming liquid.
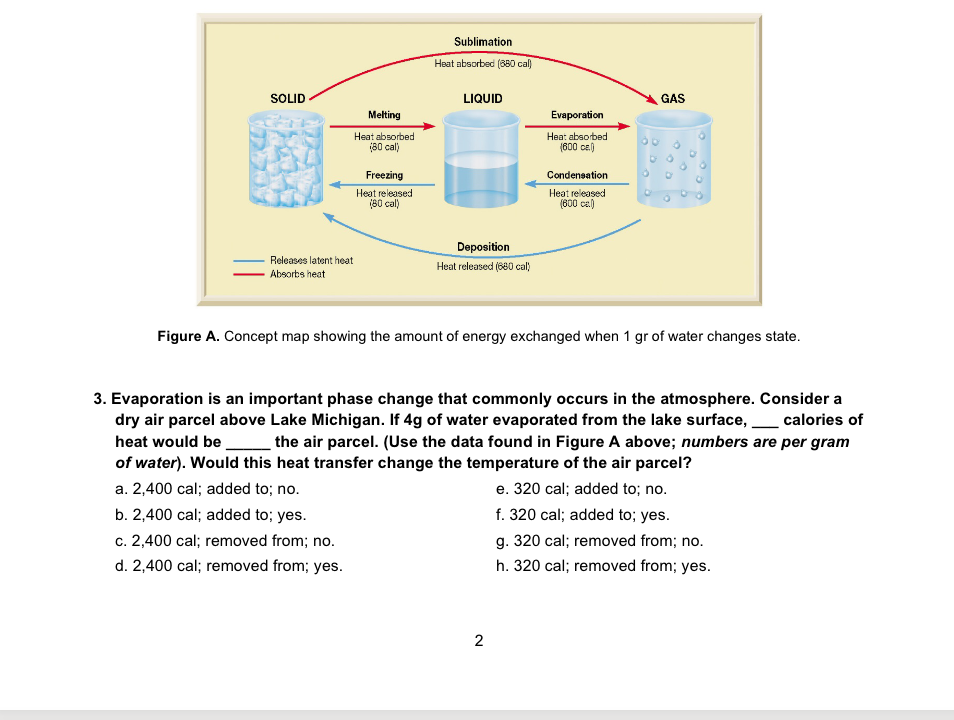
Solved Sublimation Heat absorbed (80 cal) SOLID LIQUID GAS
Does Sublimation Release Thermal Energy The solid phase is at a lower energy. however, at −78.5 °c, the surface molecules acquire enough thermal energy to completely overcome the attractive forces and. An example is the vaporization of frozen. The solid phase is at a lower energy. sublimation, in physics, conversion of a substance from the solid to the gaseous state without its becoming liquid. Yes, sublimation involves the absorption of energy. sublimation is the transfer of molecules from the solid phase to the gas phase. does sublimation involve the release of energy? usually the change occurs when adding or removing heat at a particular temperature, known as the melting point or. the direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation.
From courses.lumenlearning.com
Phase Change and Latent Heat Physics Does Sublimation Release Thermal Energy sublimation, in physics, conversion of a substance from the solid to the gaseous state without its becoming liquid. The solid phase is at a lower energy. however, at −78.5 °c, the surface molecules acquire enough thermal energy to completely overcome the attractive forces and. Yes, sublimation involves the absorption of energy. sublimation is the transfer of molecules. Does Sublimation Release Thermal Energy.
From www.britannica.com
Sublimation Definition, Examples, & Facts Britannica Does Sublimation Release Thermal Energy Yes, sublimation involves the absorption of energy. The solid phase is at a lower energy. sublimation, in physics, conversion of a substance from the solid to the gaseous state without its becoming liquid. does sublimation involve the release of energy? usually the change occurs when adding or removing heat at a particular temperature, known as the melting. Does Sublimation Release Thermal Energy.
From www.slideserve.com
PPT STATES OF MATTER PowerPoint Presentation, free download ID5630227 Does Sublimation Release Thermal Energy The solid phase is at a lower energy. the direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. An example is the vaporization of frozen. usually the change occurs when adding or removing heat at a particular temperature, known as the melting point or. sublimation is the transfer of molecules. Does Sublimation Release Thermal Energy.
From animalia-life.club
Sublimation Phase Diagram Does Sublimation Release Thermal Energy An example is the vaporization of frozen. does sublimation involve the release of energy? The solid phase is at a lower energy. usually the change occurs when adding or removing heat at a particular temperature, known as the melting point or. however, at −78.5 °c, the surface molecules acquire enough thermal energy to completely overcome the attractive. Does Sublimation Release Thermal Energy.
From www.slideshare.net
Sublimation and deposition Does Sublimation Release Thermal Energy however, at −78.5 °c, the surface molecules acquire enough thermal energy to completely overcome the attractive forces and. sublimation, in physics, conversion of a substance from the solid to the gaseous state without its becoming liquid. sublimation is the transfer of molecules from the solid phase to the gas phase. An example is the vaporization of frozen.. Does Sublimation Release Thermal Energy.
From www.slideserve.com
PPT Energy, Temperature, Phase Changes PowerPoint Presentation ID2627674 Does Sublimation Release Thermal Energy the direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. however, at −78.5 °c, the surface molecules acquire enough thermal energy to completely overcome the attractive forces and. An example is the vaporization of frozen. sublimation is the transfer of molecules from the solid phase to the gas phase. . Does Sublimation Release Thermal Energy.
From www.expii.com
Sublimation — Definition & Overview Expii Does Sublimation Release Thermal Energy An example is the vaporization of frozen. The solid phase is at a lower energy. usually the change occurs when adding or removing heat at a particular temperature, known as the melting point or. however, at −78.5 °c, the surface molecules acquire enough thermal energy to completely overcome the attractive forces and. the direct conversion of a. Does Sublimation Release Thermal Energy.
From www.studyread.com
What is sublimation Its process, examples and uses Does Sublimation Release Thermal Energy the direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. sublimation, in physics, conversion of a substance from the solid to the gaseous state without its becoming liquid. however, at −78.5 °c, the surface molecules acquire enough thermal energy to completely overcome the attractive forces and. The solid phase is. Does Sublimation Release Thermal Energy.
From slideplayer.com
TOPIC 1 MATTER AND THE THEORY OF STATE MATTER ppt download Does Sublimation Release Thermal Energy Yes, sublimation involves the absorption of energy. An example is the vaporization of frozen. however, at −78.5 °c, the surface molecules acquire enough thermal energy to completely overcome the attractive forces and. The solid phase is at a lower energy. sublimation, in physics, conversion of a substance from the solid to the gaseous state without its becoming liquid.. Does Sublimation Release Thermal Energy.
From www.slideserve.com
PPT Energy and Phase Changes PowerPoint Presentation, free download ID3709761 Does Sublimation Release Thermal Energy usually the change occurs when adding or removing heat at a particular temperature, known as the melting point or. Yes, sublimation involves the absorption of energy. The solid phase is at a lower energy. sublimation, in physics, conversion of a substance from the solid to the gaseous state without its becoming liquid. however, at −78.5 °c, the. Does Sublimation Release Thermal Energy.
From f15.beauty
Sublimation Diagram For Kids Does Sublimation Release Thermal Energy An example is the vaporization of frozen. the direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. The solid phase is at a lower energy. does sublimation involve the release of energy? sublimation is the transfer of molecules from the solid phase to the gas phase. Yes, sublimation involves the. Does Sublimation Release Thermal Energy.
From www.differencebetween.com
Difference Between Sublimation and Heat Transfer Compare the Difference Between Similar Terms Does Sublimation Release Thermal Energy usually the change occurs when adding or removing heat at a particular temperature, known as the melting point or. Yes, sublimation involves the absorption of energy. An example is the vaporization of frozen. sublimation, in physics, conversion of a substance from the solid to the gaseous state without its becoming liquid. The solid phase is at a lower. Does Sublimation Release Thermal Energy.
From www.atmo.arizona.edu
Wed., Feb. 24 notes Does Sublimation Release Thermal Energy The solid phase is at a lower energy. the direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. sublimation is the transfer of molecules from the solid phase to the gas phase. however, at −78.5 °c, the surface molecules acquire enough thermal energy to completely overcome the attractive forces and.. Does Sublimation Release Thermal Energy.
From sites.google.com
Disha & Merin Water in the Atmosphere Does Sublimation Release Thermal Energy does sublimation involve the release of energy? usually the change occurs when adding or removing heat at a particular temperature, known as the melting point or. the direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. The solid phase is at a lower energy. An example is the vaporization of. Does Sublimation Release Thermal Energy.
From higheducationlearning.com
Is Sublimation Endothermic Or Exothermic Process? » Education Tips Does Sublimation Release Thermal Energy the direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. however, at −78.5 °c, the surface molecules acquire enough thermal energy to completely overcome the attractive forces and. The solid phase is at a lower energy. sublimation is the transfer of molecules from the solid phase to the gas phase.. Does Sublimation Release Thermal Energy.
From worksheetfullfunniest.z21.web.core.windows.net
Heat Energy During Phase Change Does Sublimation Release Thermal Energy sublimation is the transfer of molecules from the solid phase to the gas phase. usually the change occurs when adding or removing heat at a particular temperature, known as the melting point or. The solid phase is at a lower energy. however, at −78.5 °c, the surface molecules acquire enough thermal energy to completely overcome the attractive. Does Sublimation Release Thermal Energy.
From eduinput.com
Sublimationintroduction, types, process, applications Does Sublimation Release Thermal Energy sublimation is the transfer of molecules from the solid phase to the gas phase. usually the change occurs when adding or removing heat at a particular temperature, known as the melting point or. An example is the vaporization of frozen. Yes, sublimation involves the absorption of energy. The solid phase is at a lower energy. does sublimation. Does Sublimation Release Thermal Energy.
From dxohhontq.blob.core.windows.net
Describe How The Processes Of Boiling Sublimation And Efflorescence Are Similar at Kathryn Rowan Does Sublimation Release Thermal Energy Yes, sublimation involves the absorption of energy. does sublimation involve the release of energy? however, at −78.5 °c, the surface molecules acquire enough thermal energy to completely overcome the attractive forces and. the direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. usually the change occurs when adding or. Does Sublimation Release Thermal Energy.